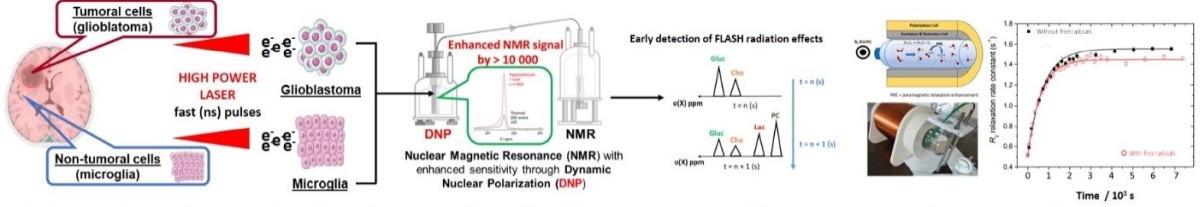
High-Energy, High-Dose Rate Radiation Generation for Advanced FLASH Radiobiology Studies
One of the research directions developed at ELI-NP focuses on pioneering experiments that utilize laser-driven radiation sources—particularly
high-energy photons - to advance FLASH radiobiology research, with a special emphasis on studying biological samples' responses to ultra-high
dose rate irradiation. Understanding cellular effects across the fundamental stages of radiation interaction—physical, chemical,
and biological—demands ultra-short, high-dose-rate exposures. Recent advances in laser and plasma acceleration technologies have made
this capability possible.
Traditional radiobiology has been limited to irradiation times ranging from microseconds to minutes, potentially impacting DNA repair processes
and altering cellular responses. In contrast, our approach leverages ultra-short (femtosecond) high-dose irradiation, allowing us to study
damage mechanisms at timescales that align closely with the DNA effects caused by free radicals generated by ionizing radiation.
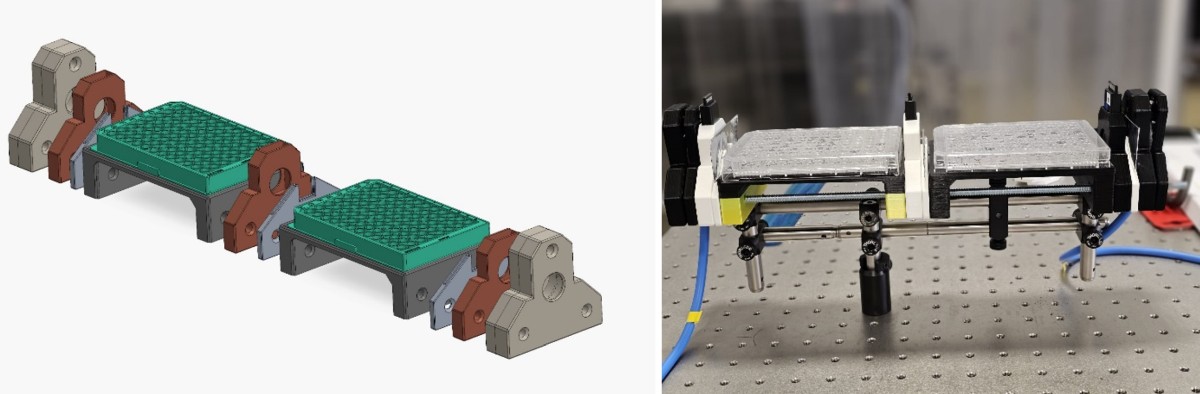
Using high-power laser systems that reach focused intensities exceeding 1022 W/cm2, we produce relativistic electrons through laser wakefield
acceleration. These electrons, accelerated to GeV energies generate high-brightness, collimated gamma, and X-ray photons via mechanisms
such as Betatron radiation, Bremsstrahlung, and Inverse Compton scattering. These photon sources, with femtosecond scale pulse durations,
can deliver precise, single-shot doses in the Gy range, essential for simulating the conditions required for FLASH radiobiology.
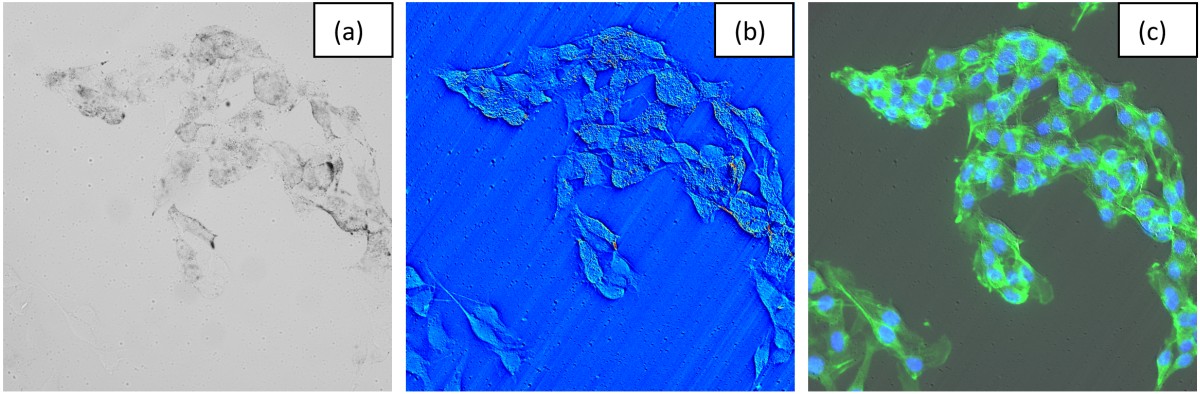
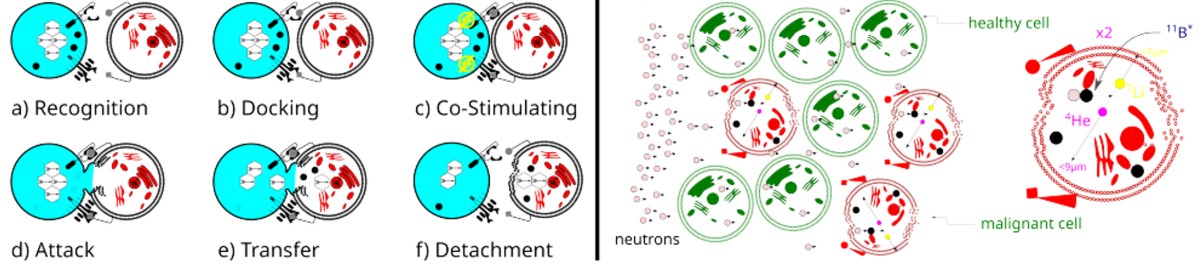
Through this method, we aim to enhance our understanding of differential cellular responses - particularly between
cancerous and healthy cells - by closely replicating radiation effects at fundamental interaction timescales.
This research represents a transformative approach in radiobiology, offering new insights into cellular responses
to extreme dose rates and paving the way for therapeutic innovations.